Q1 2025 REPORT | Q3 2025 REPORT | Q4 2025 REPORT
The CRN team was active in the second quarter, advancing its work to protect and promote the dietary supplement and functional food industry, balancing challenges and opportunities emerging in today’s unpredictable legislative and regulatory landscape, with a productive “Day on the Hill” and a thought-provoking Legal & Regulatory conference, and action on tariffs—all marking the close of a dynamic first half of the year.
Following is a brief recap of CRN’s work for its members during Q2 in each of the areas of our 2024–2026 strategic plan:
- Expanding Self-Regulatory Initiatives
- Influencing Public Policy
- Improving Consumer Access
- Cultivating Positive Public Perceptions
- Expanding Membership and Educational Offerings
Download printable one-pager here.

CRN CONVENES INDUSTRY STAKEHOLDERS, LEGISLATORS FOR HONEST, CONSTRUCTIVE CONVERSATIONS ON CAPITOL HILL
Eighty dietary supplement industry executives representing CRN member companies gathered in Washington in June for the association's annual "Day on the Hill"—a cornerstone event that promotes direct dialogue between industry leaders and lawmakers.
The day included face-to-face meetings on Capitol Hill with 70 key lawmakers and staff from both sides of the aisle, many of whom play vital roles in shaping the future of nutrition policy in the U.S. Participants advocated for expanded consumer access to dietary supplements through HSA/FSA eligibility and underscored how FDA’s current interpretation of drug preclusion creates barriers to innovation and progress.
Read more in the CRN press release and in coverage in the CRN Supplement newsletter, SupplySide Journal, and NutraIngredients-USA.

CONGRESSIONAL DIETARY SUPPLEMENT CAUCUS RE-FORMED FOR NEW SESSION
The Congressional Dietary Supplement Caucus was re-formed for the 119th Congress with the direct involvement of CRN’s govt relations team. The Caucus serves as a nonpartisan forum for Members of Congress and their staff to hear from scientific experts, healthcare practitioners, and industry stakeholders. Rep. Mike Kennedy, M.D. (R-UT-3) and Rep. Marc Veasey (D-TX-33), will serve as co-chairs. It’s first briefing of the session will occur I July on the topic of the role of dietary supplement in attaining prenatal nutrition.
NEW MEMBERS OF CONGRESS GET A CRN WELCOME
In what has become a critical biennial exercise, CRN completed its Freshmen Orientation initiative with the offices of all 79 new Members of Congress in May. The program targets new lawmakers and their healthcare staff to introduce them to the dietary supplement industry and to educate them about the regulation under DSHEA and the health benefits and economic impacts of dietary supplements. By meeting with these Congressional offices, CRN lays the groundwork for future support for policies that advance consumer access and appropriate regulation for these products.
CRN RESPONDS TO NEW TARIFFS BY ENGAGING WITH USTR, DEPT OF COMMERCE, AND WHITE HOUSE NATIONAL ECONOMIC COUNCIL OFFICIALS
CRN President & CEO Steve Mister and VP Scientific and Regulatory Affairs Luke Huber met with officials at key U.S. trade offices during the second quarter, advocating against tariffs on dietary supplements and their ingredients, highlighting the risks to consumer access, domestic jobs, the overall U.S. economy, and public health. They discussed issues outlined in CRN’s Section 232 comments with officials from the Office of Intergovernmental Affairs and Public Engagement at the U.S. Trade Representative, the Department of Commerce, and with the White House National Economic Council. CRN is committed to working with federal policymakers to ensure consumers’ continued access to safe, beneficial supplements.

CRN PARTNERS WITH NAFC TO EXPAND ACCESS TO NUTRITION FOR UNDERSERVED COMMUNITIES
 The CRN Foundation and the National Association of Free and Charitable Clinics (NAFC) formally announced their partnership under the CRN Foundation’s Access Initiative, a national effort to increasing access to high-quality dietary supplements and essential nutrition for underserved populations across the country. The initiative has already garnered significant industry support from CRN members including Nutrawise Health & Beauty, Nestlé Health Science Foundation and Vitaquest.
The CRN Foundation and the National Association of Free and Charitable Clinics (NAFC) formally announced their partnership under the CRN Foundation’s Access Initiative, a national effort to increasing access to high-quality dietary supplements and essential nutrition for underserved populations across the country. The initiative has already garnered significant industry support from CRN members including Nutrawise Health & Beauty, Nestlé Health Science Foundation and Vitaquest.
CRN EDUCATES HEALTH CARE PROFESSIONALS ON SUPPLEMENTS’ ROLE IN PRENATAL NUTRITION, DEFENDS RESPONSIBLE INDUSTRY PRACTICES FOR CRUCIAL CATEGORY
CRN championed the importance of prenatal nutrition at the 2025 American College of Obstetricians and Gynecologists (ACOG) Annual Clinical and Scientific Meeting through a special project of its foundation, made possible with support from CRN members Balchem, Church & Dwight, dsm-firmenich, Kemin, Nestlé Health Science, Niagen Bioscience, OmniActive Health Technologies, and the Unilever Wellbeing Collective.

Separately, CRN’s Government Relations team addressed legislative efforts in California that would impose new heavy metal testing and disclosure requirements for prenatal supplements. The team remains engaged with state lawmakers and other stakeholders with the goal of amending the bill during the legislative process.
CRN CONTRIBUTES TO KEY SESSIONS AT ASN's NUTRITION 2025
Presenting at NUTRITION 2025, the American Society for Nutrition’s (ASN) annual meeting, CRN SVP Scientific and Regulatory Affairs Andrea Wong, Ph.D., shared insights on key topics in prenatal health and professional development. Dr. Wong discussed developments and ongoing challenges in formulating prenatal supplements during a presentation titled “Innovations and Challenges in Prenatal Dietary Supplement Formulation: Bridging Research and Industry Practices.”
In addition, Dr. Wong served as a panelist for “Real Talk: Navigating Change in the Workplace – Fresh Perspectives, Panel Discussions, and Networking,” sharing insights on collaboration across sectors.

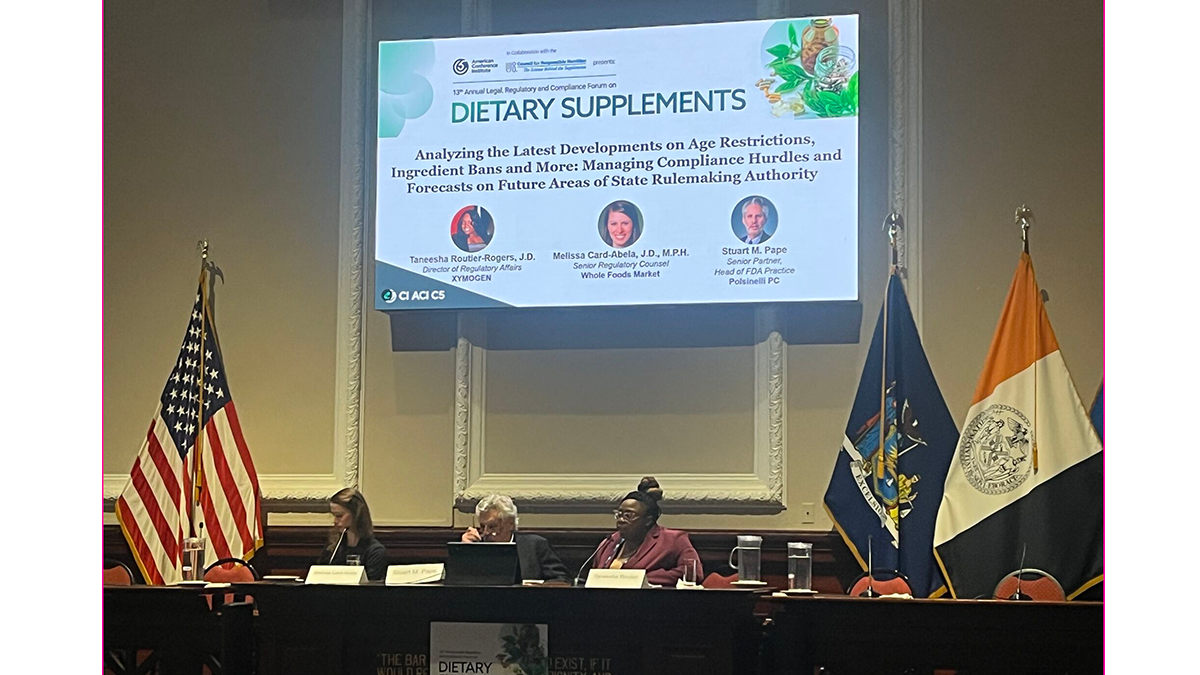
CRN PRESENTS ANNUAL LEGAL, REGULATORY, AND COMPLIANCE CONFERENCE WITH ACI
CRN convened legal, regulatory, and compliance experts for its annual forum, in collaboration with the American Conference Institute, which included a fireside chat with Cara Welch, head of FDA’s Office of Dietary Supplement Programs, along with sessions on emerging federal and state policy challenges, advertising compliance, AI innovation risks, global trade impacts, class action trends, and strategies for maintaining online and environmental compliance in a rapidly evolving regulatory landscape. Read reporting from the forum via NutraIngredients-USA.
CRN’S UPDATED EVALUATION RAISES SAFE INTAKE LEVEL FOR MAGNESIUM SUPPLEMENTS
CRN released new chapters of its Vitamin and Mineral Safety Handbook, consulted by experts worldwide, evaluating new data and raising the recommended safe upper level (UL) for magnesium supplements to 500 milligrams.
CRN FOUNDATION UPDATES ‘VITAMIN D & ME!’ WEBSITE TO HIGHLIGHT THE SYNERGISTIC ROLE OF VITAMINS D AND K2
The CRN Foundation’s “Vitamin D & Me!” website now includes details on the synergistic relationship between vitamin D and vitamin K2, addressing a knowledge gap among the public and providing new scientific context for supplement use. Read more in the full report online.
ACCESS EDUCATIONAL CONTENT ON DEMAND
CRN presented a half-day workshop, "Controlling Contaminants in Supplements: Everything You Need to Know," and webinar, "From Research to Reporting: Navigating the Communication of Supplement Science,"—both now available on demand.

GROWING THE ASSOCIATION
CRN welcomed new members in Q2, including voting members Alzchem and Vision Essence and associate member Regulatory Avenues Consulting.